Where Hearts Learn to Heal ™
We are committed to cultivating diverse health professions leaders who are dedicated to social justice and health equity for underserved populations. Outstanding education, clinical service and community engagement are at the core of the CDU experience.
Explore our programs to discover which one is right for you. Search by area of focus or level of study.



Lorem ipsum dolor sit amet, consectetur adipiscing elit
Lorem ipsum dolor sit amet, consectetur adipiscing elit
Lorem ipsum dolor sit amet, consectetur adipiscing elit
Lorem ipsum dolor sit amet, consectetur adipiscing elit


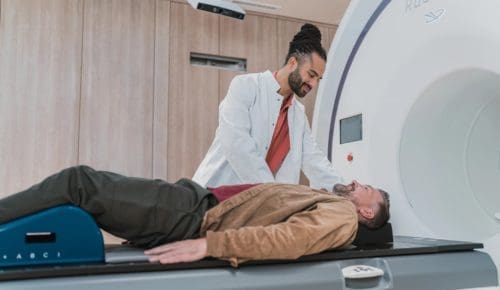




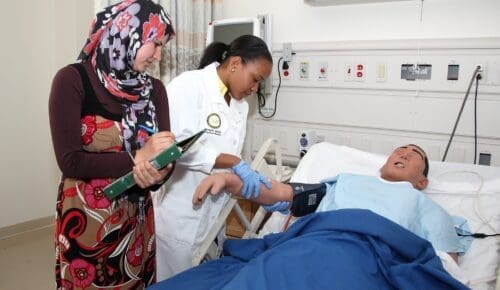


Is Charles R. Drew University right for you?
We offer a variety of degree programs for students interested in a wide variety of healthcare professions. After graduation, CDU students often return to practice and provide care in underserved communities.
Learn more about being a student at CDU.
By the Numbers
First-time pass rate for students taking the Radiologic Technology exam in 2020.
Of undergrad students received financial aid in 2021-22.
of CDU students are from communities of color.
of CDU faculty are from communities of color.
Degrees and certificates earned by the Class of 2023.

The motivation to serve. The skills to make a world of difference.

We are impacting the future of healthcare preparing diverse health professions leaders. CDU is the second-most diverse university nationally in terms of student and faculty diversity.

Our community healthcare programs, run by faculty and students, provide valuable care for vulnerable populations in the south Los Angeles area.

CDU graduates often return to underserved communities to work as healthcare providers. Ongoing research and health policy efforts focus on the root causes and the impact of health disparities.


News & Events

Charles R. Drew University of Medicine and Science (CDU) held its 9th Annual CDU President’s Breakfast at Colburn School’s Zipper Hall in downtown Los…